Our Investment in Alveo Technologies
Transforming Pathogen Detection for a Healthier, More Secure Food System
At Supply Change Capital, we back transformative technology companies that address critical challenges in our food systems. Our recent investment in Alveo Technologies represents a compelling opportunity to revolutionize pathogen detection and biosecurity in multiple industries, starting with the poultry market.
The Growing Threat
We are facing a global biosecurity crisis. The ongoing Avian Influenza (H5N1) outbreak has evolved into an unprecedented threat, spreading across birds, mammals, and now humans. In the U.S. alone, this outbreak has affected over 85 million poultry birds across 48 states since 2022 (CDC), causing billions in economic damage and driving egg prices to 45-year highs.
The CDC reports that the virus has expanded its reach beyond birds to infect at least 26 mammalian species, including dairy cattle across 12 states, creating concerns about potential adaptation to human-to-human transmission (CDC). Harvard Medical School experts warn we may be "on the cusp of a major bird flu outbreak," with the potential to become a pandemic if the virus gains the ability to spread efficiently between humans (Harvard Medical School).
Supply Change Capital Senior Advisor and Venture Partner Neil Willcocks notes, “Given the intensification of animal husbandry across the world and the continued encroachment of human activity on the remaining wilderness ecosystems, we should anticipate further zoonotic disease outbreaks and the potential for further epidemics.”
This escalating threat demands a fundamental rethinking of how we detect, track, and respond to emerging pathogens.
The Diagnostic Gap
Current molecular diagnostics remain trapped in a centralized lab paradigm:
Time: Samples must be shipped to specialized facilities, with logistical costs and hurdles to contend with, which translates into results taking days or weeks
Cost: PCR testing infrastructure requires expensive infrastructure, equipment, and trained personnel
Accessibility: Rural and resource-limited areas lack access to critical testing capabilities
Scalability: Centralized labs become bottlenecks during outbreaks, as we witnessed during COVID-19, but particularly so when contemplating reportable and foreign animal diseases such as Avian Flu
As experts at MedPage Today have highlighted, we must move beyond traditional PCR testing toward platforms that deliver "accurate and rapid diagnoses outside of traditional laboratory settings" to enable real-time decision-making where it matters most.
Alveo's Platform
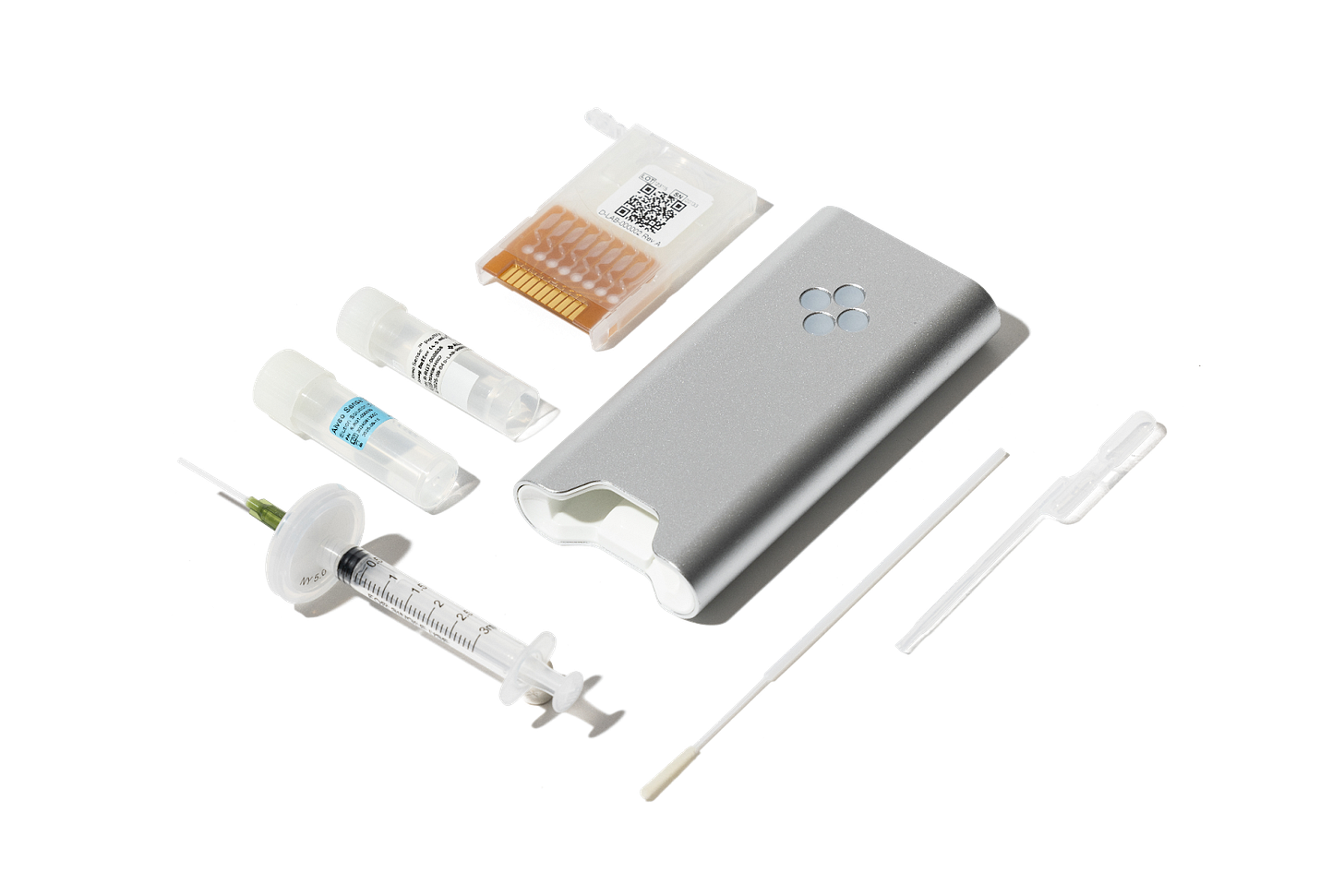
This is where Alveo Technologies delivers unprecedented value. Their IntelliSense™ platform brings molecular diagnostic precision directly to the point of need with:
Lab-quality results in under an hour (versus days/weeks for traditional methods)
PCR-comparable accuracy with 98.3% sensitivity and >99% specificity for their Avian Influenza test currently on the market internationally
Affordable testing that, over time, could enable molecular trade testing at antigen-competitive price points
Unmatched multiplexing capability of up to 7 targets per run, with 1 control
Cloud connectivity for real-time outbreak tracking and surveillance
What makes Alveo truly special is their complete ecosystem approach. Beyond the portable analyzer and test cartridges, their secure Alveo Vista™ platform provides geotagged, time-stamped results that enable real-time outbreak monitoring and response, creating a valuable data layer that traditional diagnostics cannot match.
Market Opportunity
The market opportunity is massive and multi-faceted:
Agriculture & Nutrition Testing: $23B TAM across food, agriculture, livestock, and companion animal testing
Medical Diagnostics: $51B TAM, including infectious disease testing
Industrial & Environmental: Additional multi-billion dollar opportunities
Importantly, Alveo isn't just taking share from existing markets—they’re expanding them by making molecular testing accessible in entirely new settings. The platform can detect pathogens days or weeks earlier than traditional methods, creating tremendous economic value through prevention rather than reaction and extending reach to remote locations.
Commercial Validation
Alveo's technology isn't just promising but market-ready:
CDC Partnership: The CDC selected Alveo to develop a point-of-need test for human avian influenza, providing $5.3M in funding and validation from the gold standard in pathogen detection.
Industry Leaders: Strategic partnerships with Corteva, CDC, Royal GD, and several others in the fermentation, crop, and livestock spaces demonstrate significant commercial and partnership interest.
Commercial Traction: The company secured over 100 orders/leads across 31 countries within a month of their first product launch.
Regulatory Progress: They have received approval in the Netherlands with validation underway in Italy, France, and the UK; in the USDA licensure submission process as well.
The Team
Alveo has assembled an exceptional team with deep domain expertise from companies like Thermo Fisher Scientific, Illumina, Mesa Biotech, and Abbott.
CEO Shaun Holt brings more than 20 years of operational and financial leadership experience across technology and life sciences, from startups to established companies. He served as the COO of Atonarp, Inc., an optical and mass-spectrometry instrumentation company, as CFO for Berkeley Lights, Inc., where he led the digital cell biology company through its $205M IPO, and as Head of Finance for Global Business Units & Research at Illumina, Inc., the global leader in DNA sequencing technology, where he served in a series of strategic finance leadership roles over 7+ years.
Erik Tyrrell-Knott is the Chief Business and Strategy Officer of Alveo Technologies and also brings over 20 years of experience in strategic business development for medical device and diagnostic companies. Erik most recently served as Vice President of Business Development at Atonarp, Inc, and previously as Head of Business Development for Thermo Fisher Scientific, following the acquisition of Mesa Biotech, innovator of the Accula RT PCR point-of-care testing platform, where he had served as Senior Vice President of Business Development and Product Management. Prior to Mesa, Erik served as Vice President of Business Development and Partnerships at Elsevier Corporation, and as Vice President of Business Development at GE Healthcare. He holds a Juris Doctorate from Boston College.
This is precisely the team that can help Alveo’s next phase of growth - entrepreneurs who have successfully commercialized diagnostic technologies at scale.
Our Investment Thesis
We believe Alveo Technologies represents a paradigm shift in molecular diagnostics that will:
Transform disease surveillance: Enabling early detection and containment of emerging threats
Protect food systems: Preventing devastating outbreaks that threaten global food security
Democratize precision diagnostics: Making molecular testing accessible where it's needed most
Generate actionable data: Creating valuable insights for disease monitoring and prevention
Alveo is positioned to become a category-defining company with substantial financial returns and world-changing impact. That's why we're proud to participate in the Company’s Series A extension round, joining existing investors Celesta Capital, Black Diamond Ventures, H. Barton Asset Management, and Opaleye Management in backing this exceptional team on their mission to reshape molecular diagnostics for a healthier, more secure food system.